10F, Building B, Erqi Center, Erqi District,
Zhengzhou City,
Henan Province, China
Wit:+86 15138685087
(WhatsApp/Wechat)

10F, Building B, Erqi Center, Erqi District,
Zhengzhou City,
Henan Province, China
Wit:+86 15138685087
(WhatsApp/Wechat)
Stainless steel is the most common metal, and galvanized steel is no less common than it. So, as they are both metals, what are the differences in the performance of galvanized and stainless steel? Can ss be galvanized? Is it galvanized steel? Can it be put together with galvanized steel? Can they be welded together? How to prevent galvanic corrosion between the two? How to distinguish them in daily life, and what will happen when welding them? Which one has a longer lifespan, stainless steel or galvanized steel, and what are their practical applications? Read on and you will find the answers.

Galvanized-Plates
Stainless steel plate
| Physical properties of Stainless Steel | Symbol | Value | Units |
| Thermal conductivity | k | 25 | W/M0C |
| Density | ρ | 8000 | Kg/m³ |
| Specific heat | Cp | 400 | J/kg0C |
| Solidus temperature | Ts | 1500 | 0C |
| Liquidus temperature | Tl | 1525 | 0C |
| Latent heat of fusion | Hm | 1.93*109 | J/m³ |
| Absorption coefficient | α | 1*105 | 10-1 |
| Staring temperature of Martensite | Ms | 350 | 0C |
| Austenization temperature | TA | 750 | 0C |
| Physical properties of Galvanized Steel | Symbol | Value | Units |
| Yield strength | Re | 300 | MPa |
| Density | ρ | 7833 | Kg/m³ |
| Thermal Diffusivity | α | 84.18*10-6 | M2/s |
| Specific Heat | c | 896 | J/kgK |
| Thermal conductivity | k | 65 | W/m.K |
| Ultimate tensile strength | UTS | 400 | MPa |
| Young’s Modulus | E | 210 | GPa |
| Poisson’s Ratio | G | 0.3 | τ / γ |
| Weight heat | J | 465 | kgK |
ss sheet
GI Sheet
Galvanized steel is coated with zinc to protect it from rust, while stainless steel is an alloy made with at least 10% chromium; the two have different chemical compositions, but both have the purpose of corrosion prevention.
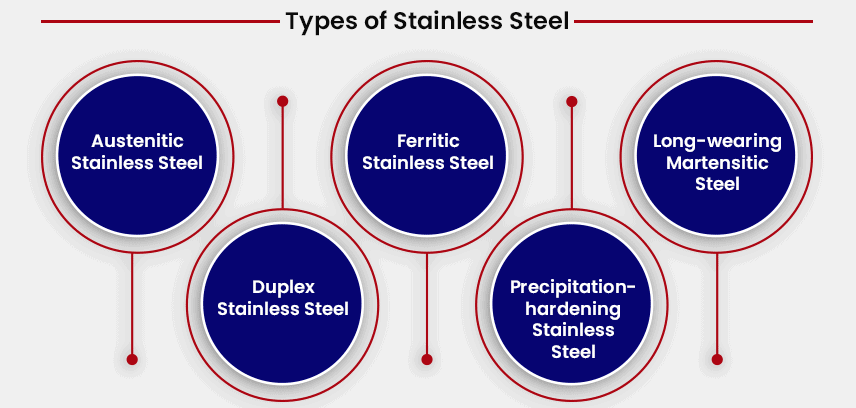
There are several types of stainless steel: Austenitic stainless steel, Ferritic stainless steel, Long-wearing martensitic steel, Duplex stainless steel, Precipitation-hardening stainless steel, each determined by the type and amount of additional materials, like chromium, as well as nickel, titanium, manganese, and/or molybdenum, present in the steel:
1. Stainless steel is the king of rust prevention and has excellent corrosion resistance, even in the atmosphere, water, acid, alkali, salt, and other media.
2. The surface is smooth, the color is bright, and it is easy to process. It can be polished, brushed, and mirrored to enhance the texture.
3. Unlike galvanized steel, its good weldability ensures the corrosion resistance of the welded joint and has excellent welding performance.
4. Its safety and hygiene level is higher than that of galvanized steel, and it is widely used in hospitals, biochemical experiments, surgical instruments, and food industries.
5. It has both strength and toughness, and its strength after alloying is higher than that of some galvanized steel. The price is more expensive than galvanized steel.
6. It can be completely recycled and reused, and it is more environmentally friendly. High temperature and low temperature do not affect its good performance.

Zero Spangle Galvanized Coils

Stainless Steel Coil
1. Galvanized steel is water-resistant, but it does not hold up well under salt water.
2. Compared to stainless steel, it’s easier to work with and much less expensive.
3. For many construction applications, galvanized steel is a trusted, economical choice, as long as it won’t be in contact with salt water.
4. Since welding removes zinc from the weld area, that area is left exposed and susceptible to corrosion. Most of the time, it is best to galvanize the steel after welding is complete.

Galvanized sheet surface
Stainless sheet surface
Stainless steel does not usually require galvanizing, but it can be galvanized in special circumstances. Since stainless steels from the 400 series do not contain nickel, they cannot be hot-dip galvanized. There are over 50 types of stainless steel, but as long as they contain some nickel in their chemistry (e.g. 300 series), they can be hot-dip galvanized.
Stainless steel products are not usually galvanized directly. Instead, the most common reason for this is that manufacturers need to weld stainless steel parts to pass galvanized steel before galvanizing them.

Galvanized stainless steel parts

Gold stainless steel cutlery
Galvanized steel and stainless steel can be used together, but the following key points should be noted to avoid galvanic corrosion.
1. Wet/salt spray environment, underwater/chemical environment.
2. Electrical isolation is recommended for the connection method. Zinc blocks are added as sacrificial anodes when conductivity is required.
3. Check the contact area for white rust every year. The inspection cycle should be shortened to 6 months in coastal areas.
Typical applications include stainless steel bolts fixing galvanized steel beams (low risk indoors), galvanized guardrails and stainless steel handrails separated by insulating sleeves, and galvanized body panels and stainless steel exhaust systems are kept apart.
It is Galvanic corrosion, also called bimetallic corrosion or dissimilar metal corrosion. It refers to two dissimilar metals, such as galvanized and stainless steel, the outer layer of galvanized steel comes into contact and is exposed to an electrolyte, such as water, one metal will corrode faster than when it is not in contact with the other.

Contact with chlorides

GI and ss steel in contact with seawater
So, it means three conditions must be met for galvanic corrosion to become a concern:
1. Multiple metals must be present with varying electrode potentials or nobility. The greater the difference, the greater the risk of galvanic corrosion.
2. These metals must be in electrical contact.
3. Exposure to an electrolyte — such as saltwater — must occur.
In the process, one metal — the anode — will corrode faster than it would alone while the other — the cathode — will corrode slower than it would alone.
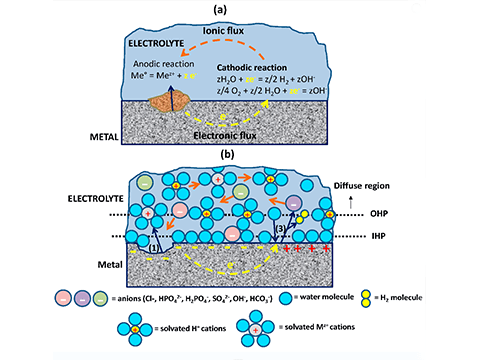
Under atmospheric conditions of moderate to mild humidity, contact between a galvanized surface and a stainless steel surface is unlikely to cause substantial corrosion. However, if the surfaces are in the presence of salt water or saltwater air, it would be best to electrically isolate the two metals.
The electrical movement between the two metals causes the stainless steel to corrode at a slower rate than normal and the galvanized steel to corrode at a faster rate than normal.
No, it is not. Galvanized steel is steel that features a zinc coating, which helps to create a barrier between the steel and the air and moisture, protecting it from rust. Stainless steel is steel that is mixed with at least 10% chromium to create an alloy that is corrosion-resistant, as well as bacteria-resistant.
When welding galvanized steel (or steel coated with a zinc-rich coating) to stainless steel, it is essential to remove the zinc from the heated zone because it is possible to get zinc into the weld, which will cause liquid embrittlement and cracking along the zinc penetration line.

Wear protection and masks

Galvanizing after welding

Protective coating
Corrosion is a key issue when we select materials and design processes. Whilst stainless steel is highly resistant to many forms of corrosion, preventing the development of corrosion is essential for long-term safe use and cost reduction.
Much of minimizing galvanic corrosion risks is simply avoiding combinations of the three elements listed above.
However, for many industries — such as those processing chemicals or operating off-shore in salt-rich environments — this is easier said than done.
This means they are less likely to suffer damage from galvanic corrosion. However, if used with highly anodic fasteners, structural elements, valves, or other components, the large difference in nobility can lead to rapid degradation of the other components.
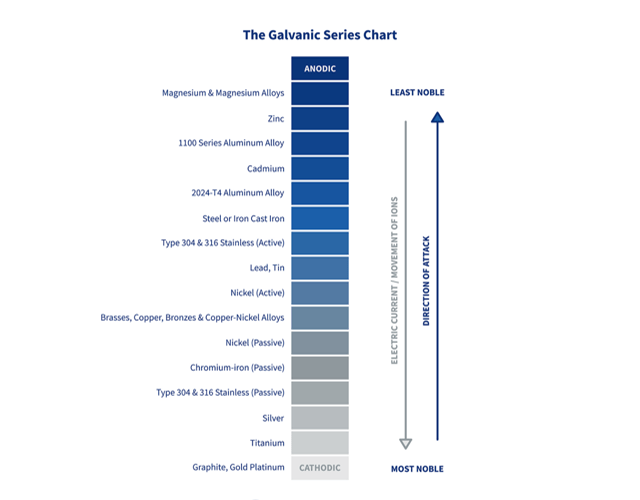
It is important to note that galvanic corrosion risks will also vary based on the electrolyte connecting both metals. For example, the risks of galvanic corrosion in very pure water are minimal. Yet, deploy the same metals in a marine or chloride-rich environment and you could see corrosion occur very rapidly.
The following chart offers examples of what galvanic corrosion risks to expect from common metal combinations.
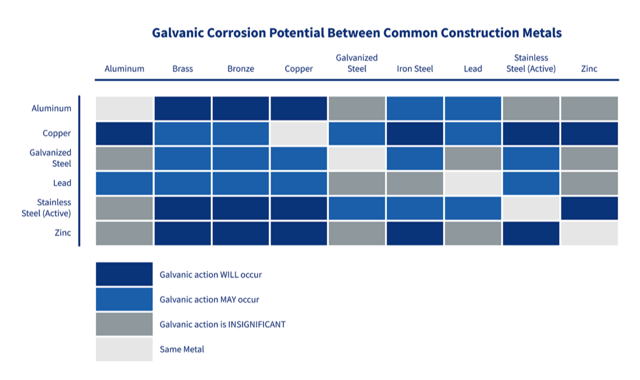
1. Insulate the different materials with a coating of non-conductive material, grease, paint, treatment, or primer. Insulating the two materials that will be in contact provides the best protection.
2. Use buffers between dissimilar metals (e.g. pipe insulation tape, gaskets, fixture linings).
3. Choose suitable connectors or fasteners, and use fasteners made of materials compatible with galvanized and stainless steel, such as copper-nickel Choose suitable connectors or fasteners, and use fasteners made of materials compatible with galvanized and stainless steel, such as copper-nickel, brass, and other corrosion-resistant materials, bolts, nuts, etc.
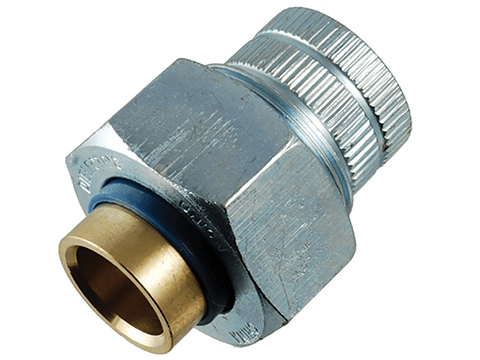
Bolts with insulating strips
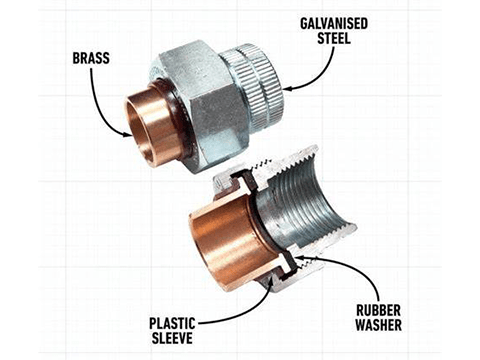
Insulation gasket included
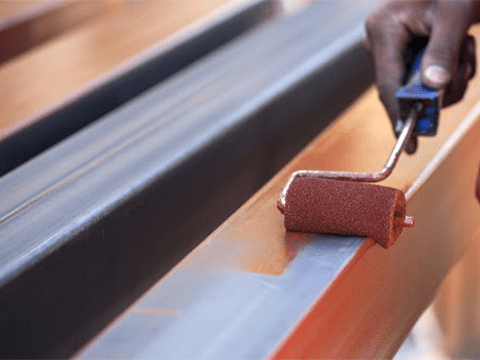
Anti-rust paint

Galvanized steel slide

Galvanized steel frame

GI steel bracket
Aerospace fan blades

Marine Applications
Pool Application

Galvanized sheet wall

Galvanized sheet metal system
If you need economy and low environmental corrosion, galvanized steel is preferred.
If you need high corrosion resistance, aesthetics, or long-term use, stainless steel is preferred.
Galvanized steel and stainless steel are two core anti-corrosion materials in modern industry, each with its own unique advantages. Galvanized steel has become the first choice in construction, infrastructure, and other fields due to its economy and reliable zinc layer protection; stainless steel, on the other hand, is irreplaceable in harsh environments such as the chemical industry and marine engineering due to its inherent corrosion resistance and high strength. When the two are used together, special attention should be paid to the risk of galvanic corrosion. Scientific means such as insulation, isolation, potential matching, or sacrificial anode can effectively extend the life of the structure.
In actual projects, it is recommended to comprehensively evaluate the material solution based on the environmental corrosion level, cost budget, and maintenance conditions. For high-demand scenarios, professional material engineers can be consulted for corrosion simulation and compatibility testing to ensure safety and reliability.
Wanzhi Steel provides a full range of galvanized and stainless steel products and technical guidance to provide the best anti-corrosion solution for your project!





