10F, Building B, Erqi Center, Erqi District,
Zhengzhou City,
Henan Province, China
Wit:+86 15138685087
(WhatsApp/Wechat)

10F, Building B, Erqi Center, Erqi District,
Zhengzhou City,
Henan Province, China
Wit:+86 15138685087
(WhatsApp/Wechat)
Not all metals contain iron, but they can rust or tarnish in other oxidizing reactions. To prevent oxidation and breakdown of metal products, such as handrails, tanks, appliances, roofing, or siding, you can choose metals that are “rust-proof” or, more accurately, “corrosion-proof.” Four basic types of metals fall into this category: Stainless steel, Aluminum, Copper, bronze, or brass, and Galvanized sheet. Today we talk further about galvanized steel.

Galvanized metal durability

Z275 GI Spangle coils
If rust is a concern, use a metal that won’t rust or one that barely rusts. For instance: stainless steel –But its price is relatively high. Coating is also a good way to maintain the good properties of the metal at a low cost. Paint is the first coating that comes to mind when looking to protect mild steel, but it doesn’t last very long. So that leads us to galvanizing, which is a really cost-effective way. Then comes the question: Does galvanized steel rust?
The short answer is, yes, and also no. Want to know why? Keep reading to find out.

Hot-Rolled-Coils-for-Sale

304Stainless Steel Plate

Wanzhi Regular Spangle GI coil
Galvanized steel is not susceptible to rust in general. Galvanizing is a commonly used method of metal corrosion prevention by covering the surface of steel with a layer of zinc to prevent the steel from coming into contact with oxygen and moisture in the external environment (e.g., air, water, etc.), thus preventing oxidation and corrosion of the steel. This zinc layer acts as a barrier, effectively slowing or stopping the rusting process of steel.

humid environments

temperate marine environment
Even if the zinc coating is scratched off, it protects nearby areas of the underlying steel through cathodic conservation and by forming a protective layer of zinc oxide. Like aluminum, zinc is highly reactive to oxygen in the presence of moisture, and the coating prevents the iron in the steel from further oxidation.

Galvanized metal before and after rusting
However, when the galvanized coating is damaged, the exposed steel substrate becomes more susceptible to corrosion. In addition, in extreme environments, such as high humidity, high salinity, or strong acids and alkalis, its ability to resist corrosion can be compromised.
Therefore, although galvanized steel is not prone to rust in general, it will eventually rust, just over a long period of time. Taking care to protect the zinc coating while in use can increase the life of galvanized steel.

Tropical marine environment

Galvanized steel under high temperature and humidity
We usually think of rust as the orange-brown flakes that form on exposed steel surfaces when iron molecules in a metal react with oxygen in the presence of water to form iron oxide. Metals may also react in the presence of acids or harsh industrial chemicals. If there is nothing to stop the corrosion, the rust flakes will continue to fall off, subjecting the metal to further corrosion until it breaks down, and rust is slowly created.
Galvanization is a zinc coating applied over the top of steel. It prevents rust and corrosion far longer than paint will, often for 50 years or more, but eventually that brown rot will set in.
Table of coefficients at different temperatures
1. Relative humidity above 60%.
2. Sodium chloride (salt) in water or air.
3. Wet or soaked environments.
4. Increase in temperature when combined with corrosive factors like humidity and industrial pollution.
5. Acids; particularly sulfur acids produced by (1) hydrogen sulfide – from volcanoes, hot springs, natural gas, and sewer gas. (2) sulfur dioxide pollution in the urban atmosphere.
6. Strong Alkalis.
7. Plasters and cement containing chlorides and sulfates.
8. Acid rainwater runoff from roofs with wood shingles.
9. Moss and lichen.
10. Contact between galvanized items and copper, pure iron, or steel causes galvanic corrosion. Galvanic (electrochemical) corrosion is an electrolytic corrosion reaction between the zinc coating and dissimilar metals when in the presence of an electrolyte such as rain, dew, fog, or condensation.
11. Acidic food and drinks.

Water containing sodium chloride
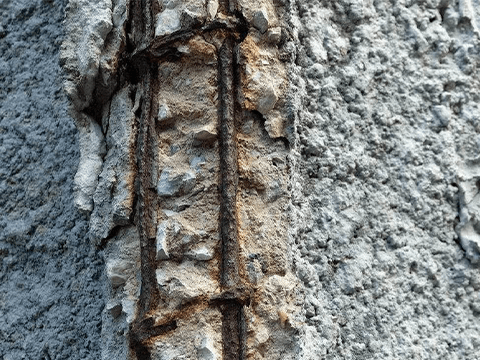
Steel in sulfate-containing cement
So how long does it take for a new galvanized steel item to rust and corrode into a useless pile of metal? It takes a long time, and if used carefully and kept dry and protected from the rain, it can last almost forever. But for those galvanized steels and tubs destined to become garden planters, landscape decorations, animal feeders, and farm water buckets corrosion is inevitable.
Galvanized steel intended for prolonged outdoor use should be hot-dipped galvanized steel; which commonly lasts for about 70 years in many different environments. It prevents rust and corrosion far longer than paint will.
The table below predicts the service life of galvanized steel based on a 30-month corrosion study conducted in 2004 on environmental factors such as humidity, moisture, and air contaminants.
| Prediction of when the zinc coating on galvanized steel will be consumed | |
| Storage of galvanized steel in wet or soaked environments | 10 years |
| 100% relative humidity | 34 years |
| Relative humidity below 60%. | 211 years |
Source: Journal of Civil Engineering Materials 2004 (11).
The corrosion resistance of zinc coatings depends mainly on the type and thickness of the coating but varies with the severity of the environmental conditions to which they are exposed (as shown in the table above). The corrosion resistance of hot-dip galvanized coatings depends primarily on the protective film (patina) formed on their surface.
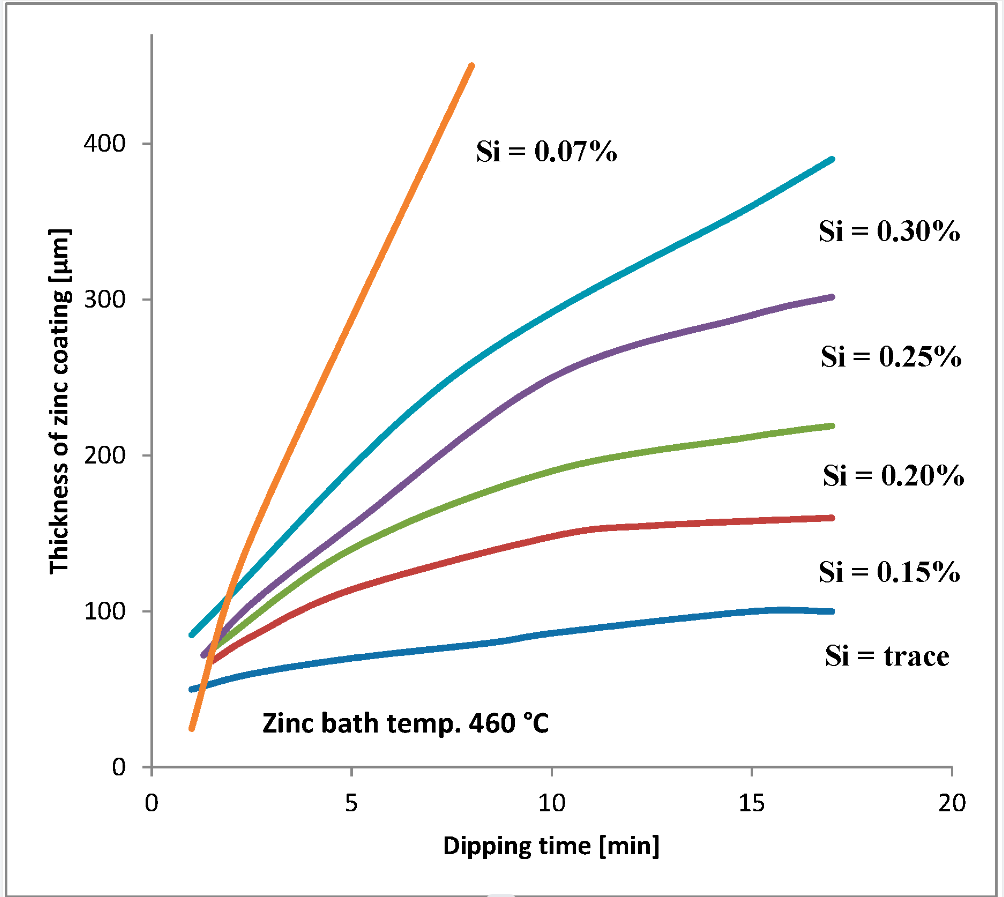
Hot-dip galvanizing time and plating chart
A 1926 study of corrosion of galvanized steel in industrial, rural, and marine areas found that:
The chart below (from the American Galvanizers Association) illustrates how long it takes for galvanized steel to rust at different zinc thicknesses.
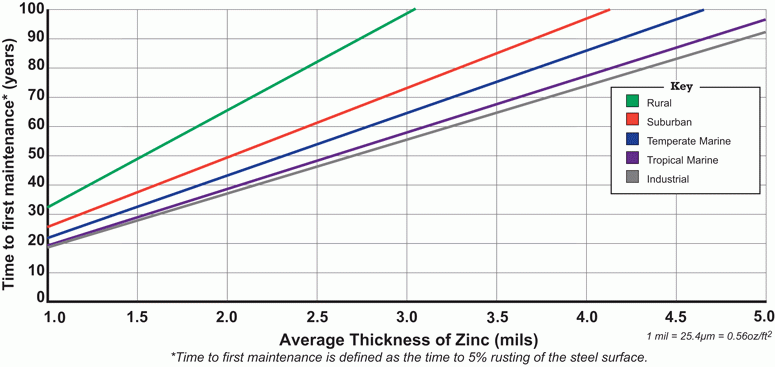
Galvanized-steel-last-by-environment
The thicker the zinc coating, the longer the galvanized steel will resist corrosion. The thickness of the zinc is shown along the horizontal axis, as shown above, and it was noted in the 1926 study that for each location, the rate of corrosion varies essentially with time.
Local conditions are important considerations when you’re thinking about using galvanized steel. Specifically:
The presence of sulfur dioxide in the atmosphere greatly affects the rate of corrosion of zinc in the atmosphere. When acids (pH below 7) attack the galvanized layer, the pH of the steel surface decreases and the rate of corrosion increases.
It has been observed that mist and dew from industrial sites have pH values as low as 3.
Thus, the reason for the more corrosive nature of industrial atmospheres is the presence of acidic sulfur dioxide pollutants in the air.

Use in humid environments

Soil acid plating affects the corrosion rate
A galvanizing corrosion study published in 2015 found that sulfur dioxide, dust, humidity and carbon dioxide have the greatest impact on corrosion. Concentrations of these pollutants peak during the winter months, when fossil fuel combustion increases. Additionally, airborne chlorides can affect corrosion rates.
Just as the PH of the atmosphere affects the rate of corrosion, so does the acidity of the soil. Hot-dip galvanizing can last 35 to 50 years in the harshest soils, such as those in which it is buried (e.g., at the base of a fence post). In less corrosive soils it can last 75 years or more.
In summary, the degree of exposure depends largely on the type of soil and overall conditions (muddy and wet or sandy or dry).

Moist soil

Sandy soil

Extremely dry soils
If the melting point of zinc is below 787°F (420°C), high temperatures alone will not break down the zinc layer. However, when combined with corrosive factors such as humidity and industrial pollution, high temperatures become an accelerating factor. Low temperatures have no effect on the zinc coating.

GI metal in temperatures below -40 F
Polar environments
The temperature itself has relatively little effect on the relative humidity. Galvanized coatings respond well to very cold and very hot temperatures. For hot dip galvanized coatings, there is no significant difference in corrosion rates at temperatures below -40 F, as stated in the first point, but at higher temperatures, the zinc is affected.
Industrial wastewater environment

Suburban use of galvanized steel
According to a publication by American Galvanizers, the recommended maximum temperature, if exposed continuously for a long period of time, is 392 F. Such high temperatures will cause the outer zinc layer of the galvanized metal to flake off from the zinc alloy layer. Although the remaining zinc alloy layer will provide corrosion protection for the steel, the protection time is shortened when compared to an intact free zinc layer on the exterior.

Unaffected in low temperatures
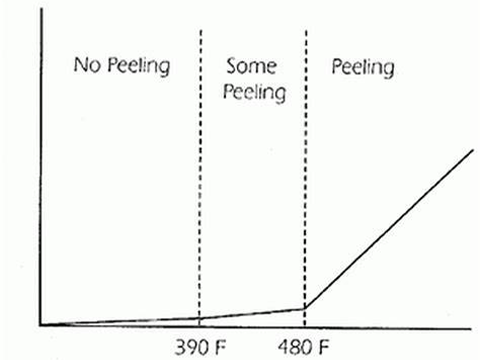
Zinc diffusion rate
Therefore hot dip galvanizing continues to be used to ensure long-lasting and maintenance-free corrosion protection. It’s as if the hot-dipped galvanized metal of Wanzhi can defy the elements.
The following are listed in order of environmental corrosiveness from strongest to weakest:
1. Industrial Environment ☆☆☆☆☆
2. Tropical Marine Environment ☆☆☆☆

In urban area

Suburban area
3. Temperate marine environment ☆☆☆
4. Suburban environments ☆☆
5. Rural environments ☆
Although galvanized steel will not last indefinitely, it is an unmatched corrosion-resistant metal. It corrodes slowly enough that it’s not a big deal, which is why galvanized steel has been used for the past 2000 years and why it may be right for your application.

peri-urban environment

Rural environments
However, it is worth noting that there is another good way to apply a protective coating such as paint to galvanized steel will alleviate the problems caused by corrosion of the zinc coating, such a finished product is called color-coated steel coil. They are available for sale at Wanzhi.
Galvanized steel is becoming the preferred material in the fields of construction, transportation, energy, etc., due to its excellent anti-corrosion performance and ultra-long service life. As an industry-leading galvanized steel supplier, Wanzhi Steel always insists on innovation-driven quality:
✔ Adopting the fully automatic galvanizing production line of SMS Group of Germany to ensure the stable performance of products.
✔ Strictly implement the ISO 9001 quality management system, and each batch of products can be traced.
✔ Provide galvanized steel solutions for more than 120 countries around the world, with a total of more than 10,000 service projects.
✔ 24-hour professional customer service team, providing full-process services from material selection suggestions to after-sales support.
Contact Wanzhi Steel now to get exclusive quotes and technical solutions! Our galvanized steel experts are ready to provide you with: 1-on-1 material selection guidance, flexible customized services, and global logistics solutions.





